Funding review process and committees
Discover how our funding schemes are reviewed and who is on each committee panel.
We're a member of the Association of Medical Research Charities (AMRC) who provide leadership, governance and monitor compliance in funding high-quality, world-class research. All our research is funded in line with the AMRC principles and guidelines.
Our general funding review process:
Our rigorous application review process varies slightly between our different funding schemes but broadly follows the process outlined below. Details of the specific process for each scheme will be outlined in the guidance document for that particular scheme.
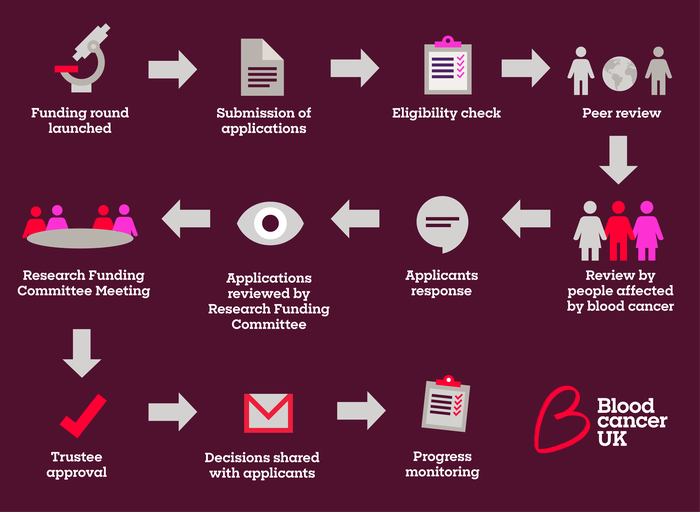
A general overview of our research funding process. Please note that the process will differ slightly depending on the funding call.
1) Peer review and response
Applications to our funding schemes are usually reviewed by independent medical and scientific expert (peer) reviewers, people affected by blood cancer and members of our funding or review committees.
Applicants are then given the opportunity to respond to the reviewers' comments before their application is considered by members of the funding committee.
2) Funding committee review
At the funding committee meeting, committee members and people affected by blood cancer discuss, review and score the applications based on scheme specific criteria. Applications are ranked by the average score they receive from those present. The set of applications that are ranked as fundable are shared with our Board of Trustees for discussion and approval.
Our funding committees consider different categories of application which you can read below. Where we require specific expertise, members of one committee can be co-opted to another committee for the assessment of a specific scheme.
Research Funding Committee
- Overall responsibility: reviewing and recommending research for funding for a portfolio of awards across the full research pipeline
- Core responsibility: Project Grants and Innovative Pilot Grants
- Membership: Research scientists and clinicians.
- Professor Tim Somervaille (Chair), Cancer Research UK Manchester Institute, University of Manchester
- Professor James Allan, Newcastle University
- Professor Lesley Anderson, University of Aberdeen
- Professor Duncan Baird, Cardiff University
- Professor Ronjon Chakraverty, University of Oxford
- Professor Mark Cragg, University of Southampton
- Dr Mike Chapman, University of Cambridge
- Professor Claire Edwards, University of Oxford
- Dr Amir Enshaei, Newcastle University
- Dr Miguel Ganuza, Queen Mary University of London
- Professor Florian Grebien, University of Veterinary Medicine Vienna
- Professor Olaf Heidenreich, Princess Maxima Centre Utrecht
- Professor Ian Hitchcock, University of York
- Dr John Jones, Brighton and Sussex Medical School
- Dr Farhat Khanim, University of Birmingham
- Professor Ulf Klein, University of Leeds
- Professor Jan Henning Klusmann, Goethe University Frankfurt
- Professor Kamil Kranc, Institute of Cancer Research, London
- Professor Adam Mead, University of Oxford
- Dr Thomas Milne, University of Oxford
- Dr Charlotte Pawlyn, The Institute of Cancer Research
- Dr Elspeth Payne, University College London
- Dr Bethan Psaila, University of Oxford
- Professor Vikki Rand, Teeside University
- Dr Anupama Rao, Great Ormond Street Hospital for Children NHS Foundation Trust
- Dr Neil Rodrigues, University of Cardiff
- Professor Ingo Ringshausen, University College London
- Professor Tatjana Stankovic, University of Birmingham
- Professor Jonathan Strefford, University of Southampton
- Professor Michelle West, Queen Mary University of London
- Professor David Westhead, University of Leeds
- Dr Adam Wilkinson, University of Oxford
- Dr Bela Wrench, Queen Mary University of London
Clinical Trials Review Committee
- Overall responsibility: providing scientific validation and quality assurance for Blood Cancer UK through funding, progress review, and management of a portfolio of clinical research awards where clinical research makes up the largest component of the research planned, or any infrastructure awards that support clinical research in line with our research strategy.
- Core responsibility: Transformational Research Awards and other clinical schemes.
- Committee members: The committee members are experts in late stage translational and clinical research and trial design and delivery across a spectrum of conditions and treatment modalities. At least 20% of the membership of the Committee have expertise in clinical study statistics and methodologies.
- Professor Martin Kaiser (Chair), The Institute of Cancer Research
- Professor Christopher Fox (Deputy Chair), Nottingham University Hospitals NHS Trust
- Professor Rachael Hough, University College London Hospitals NHS Foundation Trust
- Angela Casbard, Cardiff University
- Professor Mhairi Copland, University of Glasgow
- Professor Steven Knapper, Cardiff University
- Dr Tobias Menne, Newcastle upon Tyne Hospitals NHS Foundation Trust
- Aimee Jackson, University of Birmingham
- Dr Donal McLornan, University College London Hospitals NHS Foundation Trust
Fellowships Committee
- Overall responsibility: funding recommendations, review and management of Blood Cancer UK’s fellowships and career support activities.
- Core responsibility: Early Career Advancement Fellowships
- Committee members: the membership includes both research scientists and clinicians.
- Professor Katrin Ottersbach, University of Edinburgh
- Professor Matthew Collin, Newcastle University
- Dr Sarah Dimeloe, University of Birmingham
- Professor Martin Dyer, University of Leicester
- Dr Alanna Green, University of Sheffield
- Dr William Grey, University of York
- Professor Vignir Helgason, University of Glasgow
- Dr Sophie Kellaway, University of Nottingham
- Professor Ghulam Mufti, King’s College London
- Dr Lydia Lee, University College London
- Dr Piers Patten, King's College London
- Dr Ali Roghanian, University of Southampton
- Professor Alex Tonks, Cardiff University
- Professor Suzanne Turner, University of Cambridge
- Professor Anindita Roy, University of Oxford
- Dr Simona Valletta, University of Manchester
3) Trustee approval
Recommendations for all awards made by our funding committees are sent to our Board of Trustees, who make the final decision on which applications we will fund. The Trustees’ decision is final and non-negotiable.
The Blood Cancer UK Research Team will provide feedback on all applications and will include the committee’s evaluation. To ensure fairness and to protect the integrity of the funding process, committee members cannot discuss any decisions with applicants and therefore members should not be approached.
More information and get in touch
If you have any questions about the review process for a specific funding scheme, please contact us at [email protected].
How we manage conflicts of interest in funding decisions
We seek to identify and minimise actual, potential or perceived conflicts of interest when individuals are providing expertise and advice to the charity that influences or could be seen to influence application review, funding decisions and post award monitoring.
We screen all external peer and patient reviewers and committee members for any potential conflict of interest before we send an application to them. We also ask these reviewers and members to declare any conflicts of interest they may have with the application or applicant/s which we might not know about.
Download our conflict of interests in funding decisions policy below to find out more.
