How does acute promyelocytic leukaemia begin?
Professor Ellen Solomon and the late Professor David Grimwade received Blood Cancer UK funding to investigate how acute promyelocytic leukaemia (APL) develops. Here's a breakdown of what they discovered.
One hallmark of APL is the disruption of tiny protein structures called nuclear bodies, which are found in the nucleus, where the genetic information of a cell is stored. In their exciting new research, Professors Solomon and Grimwade have shown that a fault in PML, a gene that makes a protein found in the nuclear bodies, can cause the nuclear bodies to disintegrate.
Together with a fault in another gene called RARα, this can kick-start APL. This better understanding of how it begins will help researchers find new ways to treat APL.
What is APL and how is it treated?
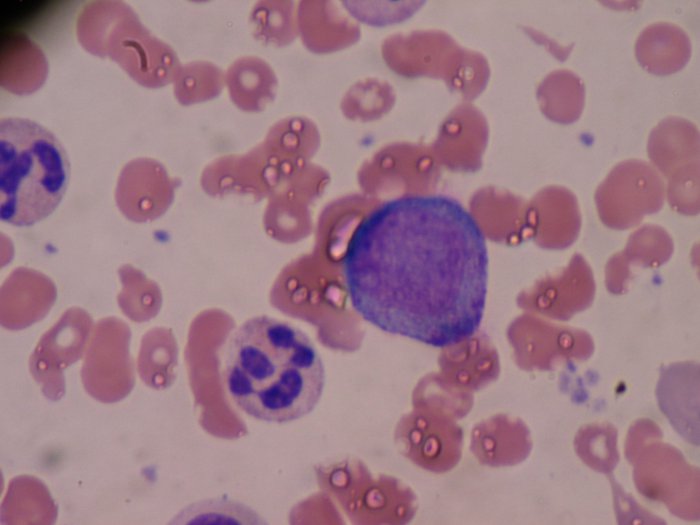
APL is a type of blood cancer that happens when the bone marrow makes too many of a type of an immature white blood cell called a promyelocyte. Promyelocytes normally develop into mature white blood cells called granulocytes, which help defend the body against infection. The solid purple cell in the below image is the centre of a promyelocyte. Promyelocytes multiply out of control in acute promyelocytic leukaemia.
There are around 170 cases of APL each year in the UK. Almost all of them start with a genetic error that fuses together the two genes RARα and PML. This fusion generates an abnormal fusion protein called PML-RARα, which blocks the ability of promyelocytes to become granulocytes.
Unlike many other types of blood cancer, APL has recently become relatively easy to treat. As well as standard chemotherapy, doctors use a drug called ATRA that forces promyelocytes to develop into granulocytes. The extra granulocytes will then die off, but people with APL need to have maintenance ATRA and standard chemotherapy for another two years after the cancer is gone to prevent it from returning.
Of course, chemotherapy has many unpleasant side-effects, such as tiredness, vomiting and being more prone to infections. ATRA can also cause a different set of side-effects known as ‘retinoic acid syndrome’, including fever, low blood pressure and shortness of breath.
It is therefore very important to find other treatments for APL that do not have such severe side effects. One way to do this is to try to catch the leukaemia as it develops rather than when it is full-blown.
Fortunately, the time between the initial genetic damage and the development of full-blown APL after further genetic damage is very long, so there are plenty of opportunities to catch the process as it unfolds. One of these opportunities is an early change that happens to a part of the cell called the ‘nuclear bodies’, which Professors Grimwade and Solomon and their team have been investigating.
Inside APL cells
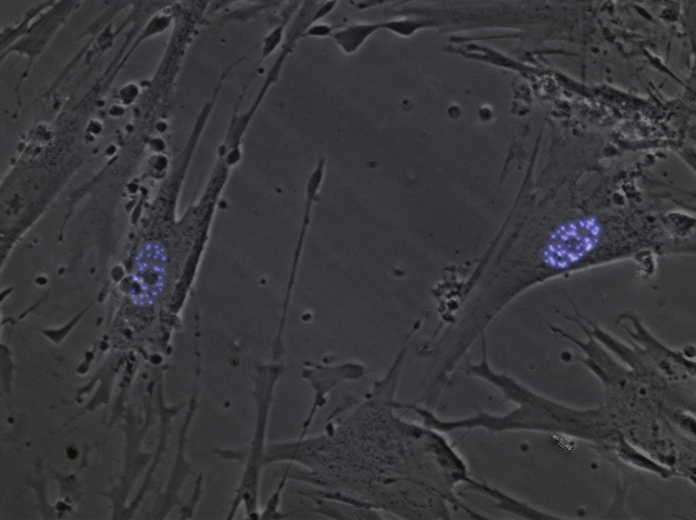
In healthy cells, the normal PML gene (the same one that gets fused with the RARα gene in APL) contains instructions to make a normal PML protein, which is involved in many of the cells’ tasks. One of these tasks is to create nuclear bodies (very small structures that may act as ‘storage depots’ for unneeded proteins in the cell), although their exact role is not clear. Damage to the PML gene causes nuclear bodies in APL cells to disintegrate into even smaller structures called nuclear microspeckles.
In the image below, the purple areas are nuclear bodies, which break down into nuclear microspeckles in APL. Professors Grimwade and Solomon wanted to know whether nuclear microspeckles can kick-start leukaemia, or whether other things also need to happen for APL to begin.
Do nuclear microspeckles cause APL?
To test the idea that nuclear microspeckles could start APL, the researchers genetically modified mice. The mice could have a fault in the PML gene (which causes nuclear microspeckles), in the RARα gene, or in both genes. They then checked whether the mice developed leukaemia over the next 18 months. None of the mice which had a fault in just the PML gene developed leukaemia, but the researchers found leukaemia in about one in twenty of the mice with a fault in just the RARα gene, and more than one in ten of the mice with a fault in both genes.
Their findings indicate that nuclear microspeckles on their own are not enough to cause APL, but when combined with damage to the RARα gene, they can make leukaemia more likely – this is the exact combination of damage that is seen in almost all people with APL. Excitingly, the researchers have also found that ATRA, one of the drugs used to treat APL, causes the nuclear microspeckles to come back together into nuclear bodies (previously, it was not clear how ATRA worked).
The cascade effect of DNA damage in APL
Looking at the genetic material of cells taken from mice that developed APL showed that damage to the nuclear bodies caused by the PML protein fault could also damage the DNA’s ability to repair itself by blocking other proteins from doing their jobs.
The researchers identified four specific proteins in the mice (53BP1, Brca1, B1m and Rad51) that have roles in the cell that are likely to be disrupted by the fault in the PML protein. All of these proteins are involved in DNA damage repair. We now know that APL probably happens through a cascade effect involving damage to these four proteins, which moves the cell’s activities further and further away from a healthy state unless the damage is repaired.
What do these findings mean for people with APL?
We now have a better understanding of how APL begins, which can help future research on stopping APL before it fully develops. This knowledge will also help researchers working on other types of leukaemia where DNA damage repair goes wrong.
The four specific genes that have been identified as being likely to make it difficult for mouse APL cells to repair themselves are also present in humans. This means that those genes are likely to be involved in human APL as well. This will need further testing, but they may be useful targets for new drugs that can stop APL in its early stages.
Essential for understanding leukaemia
Professor Solomon said: “Previously it was difficult to determine whether PML or RARα contributed to the initiating events of APL. We now know that disrupted PML plays a key role in APL development. The additional finding of its causing disruption in repair of DNA damage is essential for understanding this leukaemia in further detail, as well as other cancers.”
You can read Professors Grimwade and Solomon’s original article in the journal Blood (abstract free, full paper behind paywall).
Find out more about acute promyelocytic leukaemia (APL).
Blood Cancer UK funding award
Professors Solomon and Grimwade, both at King’s College London, received a Blood Cancer UK funding award in 2013 for their work on understanding the drivers of APL.